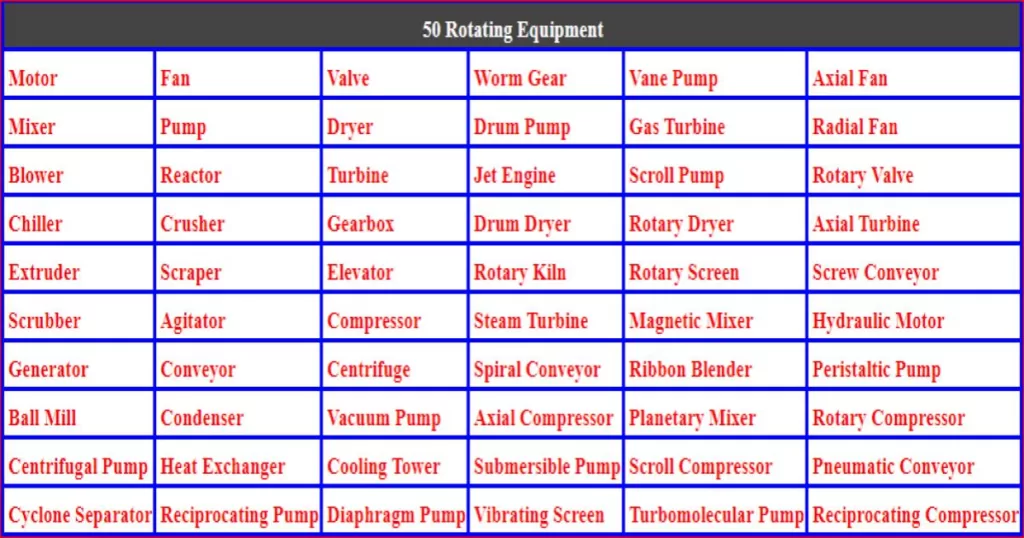
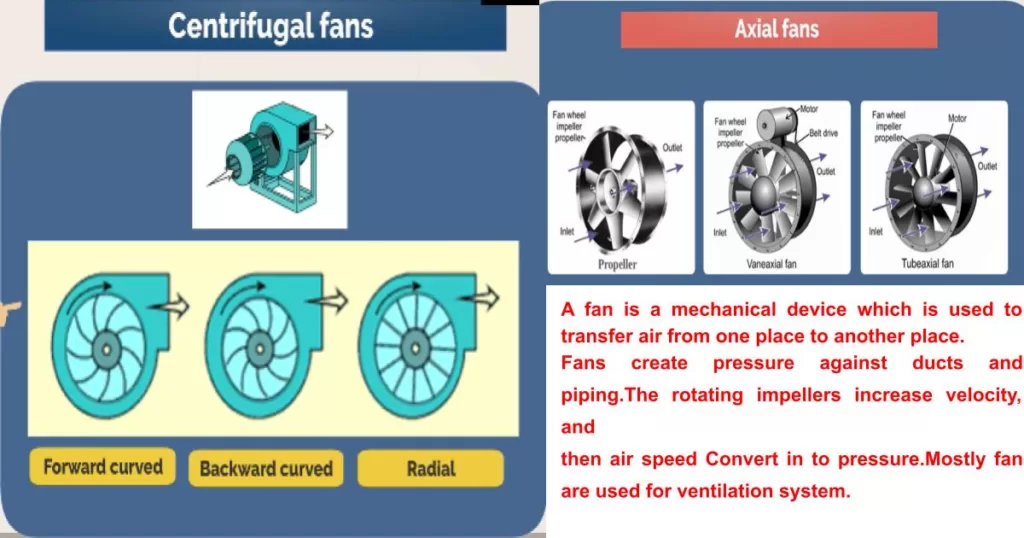
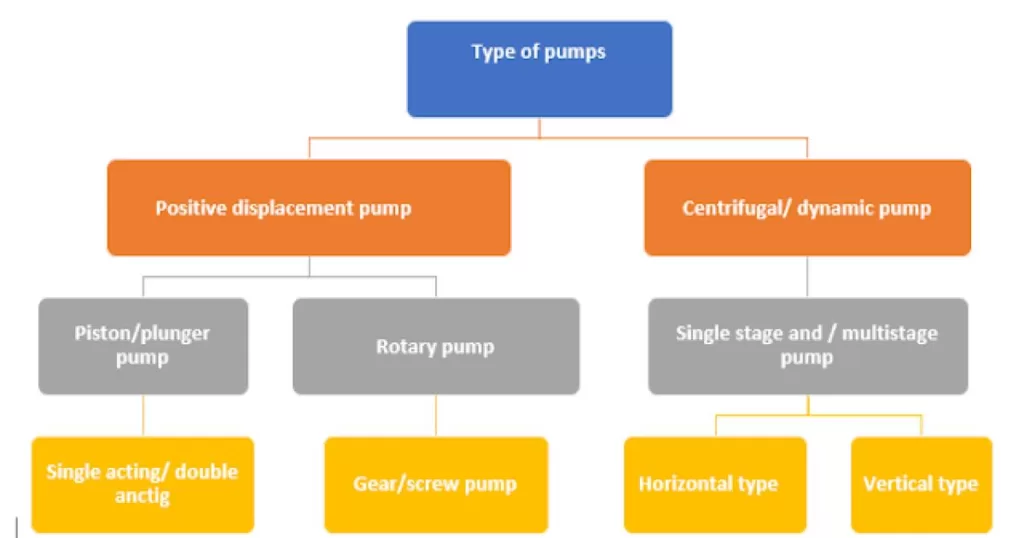
Chemical Plant Chronicles: 50 Key Concepts
Key differences in Chemical Plant Chronicles is important for several reasons. Chemical plants involve the handling and processing of various chemicals, some of which can be hazardous. Knowing the specific features and processes of each plant is crucial for ensuring the safety of workers, the community, and the environment. Differences in safety protocols, equipment, and chemical handling procedures may exist among different plants.
Material Balance vs. Energy Balance:
Material balance and energy balance are two concepts commonly used in engineering and science, particularly in fields like chemical engineering and thermodynamics. Here are brief definitions for each:
Material Balance Definition:
A material balance and mass balance term are use interchangable, that is a systematic accounting of mass entering and leaving a system. It involves tracking the flow of substances (e.g., mass, chemicals, components) into and out of a system and is based on the principle of conservation of mass. In a closed system, the total mass remains constant, and the material balance equation is expressed as the sum of inputs equal to the sum of outputs.
Energy Balance Definition:
An energy balance is a systematic approach to accounting for energy entering and leaving a system. It is based on the principle of conservation of energy, which states that energy cannot be created or destroyed, only transferred or converted from one form to another. In an energy balance, the total energy content of a system remains constant. The balance equation accounts for the various forms of energy, such as heat, work, and internal energy, as they flow into and out of the system.
Solvent vs. Solubility:
Solvent Definition: A solvent is a substance, typically a liquid, capable of dissolving other substances to form a homogeneous mixture. It is the component of a solution that is present in the largest quantity.
Solubility Definition: Solubility refers to the maximum amount of a substance (solute) that can dissolve in a specific solvent under given conditions, such as temperature and pressure. It is usually expressed in terms of grams of solute per 100 grams of solvent.
Thermoplastics vs. Thermosetting Plastics:
Thermoplastics: Thermoplastics are a type of polymer that can be melted and re-molded multiple times without undergoing any significant chemical change. They are characterized by their ability to soften when heated and solidify when cooled, making them highly versatile for various manufacturing processes. Common examples of thermoplastics include polyethylene, polypropylene, PVC (polyvinyl chloride), and polystyrene.
Thermosetting Plastics: Thermosetting plastics, on the other hand, are polymers that undergo a chemical change during the curing or molding process, irreversibly setting into a hardened form. Once these plastics are set, they generally cannot be melted and re-molded. The curing process involves the formation of strong cross-linked bonds, resulting in a rigid and durable material. Examples of thermosetting plastics include epoxy, phenolic, and melamine.
Feedwater vs. Boiler Water:
Feedwater:
Definition: Feedwater refers to the water that is supplied or fed into a boiler to be converted into steam. This water serves as the raw material for steam generation and is typically treated to remove impurities that could lead to scale formation, corrosion, or other issues in the boiler. Proper treatment of feedwater is crucial for the efficient and safe operation of boilers, as it helps to prolong the life of the boiler and maintain optimal performance.
Boiler Water:
Definition: Boiler water is the water within a boiler that is heated to produce steam. It is the result of the heat transfer process from the feedwater to the working fluid (steam) within the boiler. The quality of boiler water is important for the overall health and efficiency of the boiler system. Contaminants or impurities in the boiler water can lead to problems such as scale formation, corrosion, and reduced heat transfer efficiency. Regular monitoring and treatment of boiler water are essential to ensure the proper functioning of the boiler and to prevent damage to the system.
Naphtha vs. Diesel:
Naphtha and diesel are both hydrocarbon-based fuels, but they differ in their properties and uses.
Naphtha:
Definition: Naphtha is a liquid hydrocarbon mixture that falls within the range of light distillates. It is typically produced through the distillation of crude oil or the refining of petroleum. Naphtha is a versatile feedstock in the petrochemical industry and is used as a solvent, in the production of gasoline, and as a component in the manufacturing of various chemicals.
Diesel:
Definition: Diesel is a heavier distillate derived from crude oil through the refining process. It consists of hydrocarbons with higher molecular weights compared to naphtha. Diesel fuel is commonly used as a fuel for internal combustion engines in vehicles, machinery, and industrial applications. It is characterized by its higher energy density and lower volatility compared to gasoline.
Hazop Analysis vs. FMEA (Failure Modes and Effects Analysis):
HAZOP (Hazard and Operability Study) Analysis and FMEA (Failure Modes and Effects Analysis) are both systematic methods used in the field of risk management and safety engineering, but they focus on different aspects of systems. Here are brief definitions for each:
HAZOP (Hazard and Operability Study) Analysis:
HAZOP is a systematic and structured process used to identify potential hazards, deviations, and operability issues in a system, process, or facility. It involves a multidisciplinary team that systematically examines each element of the system to identify possible deviations from the intended design or operation. The primary goal is to assess and mitigate risks associated with both process safety and operability.
FMEA (Failure Modes and Effects Analysis):
FMEA is a systematic method for evaluating and prioritizing potential failure modes in a system, product, or process. It involves identifying possible failure modes, determining their causes and effects, and assigning a risk priority number (RPN) to prioritize the most critical issues. FMEA is widely used in various industries to enhance reliability, prevent failures, and improve the overall performance of systems.
Fluidized Bed Reactor vs. Fixed Bed Reactor:
Fluidized Bed Reactor: A reactor where a bed of solid particles is suspended and behaves like a fluid, providing efficient contact between reactants and catalyst.
Fixed Bed Reactor: A reactor where the catalyst is fixed in place, and reactants flow through the catalyst bed.
Exothermic Reaction vs. Endothermic Reaction:
Exothermic Reaction: A chemical reaction that releases heat to its surroundings.
Endothermic Reaction: A chemical reaction that absorbs heat from its surroundings.
Saturated vs. Superheated Steam:
Saturated Steam:
Saturated steam is steam that exists at the same temperature as its boiling point or saturation temperature for a given pressure. At this point, any additional heat energy added to the saturated steam will result in the steam undergoing a phase change into water and steam coexisting. Saturated steam is commonly used in various industrial processes, such as in power generation and heating applications.
Superheated Steam:
Superheated steam is steam that has been further heated beyond its saturation point. In other words, it is steam that exists at a temperature higher than its boiling point for a specific pressure. The process of superheating increases the energy content and temperature of the steam, making it suitable for specific industrial applications where high-temperature steam is required. Superheated steam is often used in power plants, industrial processes, and certain types of steam engines.
Vapor-Liquid Equilibrium vs. Liquid-Liquid Equilibrium:
Vapor-Liquid Equilibrium (VLE):
Definition: VLE refers to the state of equilibrium between a vapor phase and a liquid phase in a system. This equilibrium occurs when the rate of evaporation of a substance from the liquid phase equals the rate of condensation of its vapor phase under a specific set of conditions such as temperature and pressure. In simpler terms, it is the equilibrium between the gas and liquid forms of a substance.
Liquid-Liquid Equilibrium (LLE):
Definition: LLE, on the other hand, involves the equilibrium between two immiscible liquid phases in a system. Immiscible liquids are those that do not readily mix or dissolve in each other. The equilibrium is established when the chemical potentials of the components in both liquid phases are equal under specific conditions like temperature and pressure. Liquid-liquid equilibrium is commonly observed in systems containing two distinct liquid phases, each rich in different components.
Corrosion Resistance vs. Abrasion Resistance:
Corrosion Resistance:
Definition: Corrosion resistance is the ability of a material to withstand the detrimental effects of chemical reactions with its environment, particularly those caused by corrosive substances such as acids, salts, or moisture. Materials with high corrosion resistance are less prone to degradation or deterioration due to these chemical reactions.
Abrasion Resistance:
Definition: Abrasion resistance refers to the ability of a material to resist wear, erosion, or rubbing action caused by the mechanical interaction between surfaces. Materials with good abrasion resistance can withstand the gradual removal of material or surface damage when subjected to friction, rubbing, or scraping.
API Gravity vs. Specific Gravity:
API Gravity:
Definition: API gravity, or the American Petroleum Institute gravity, is a measure of how heavy or light a petroleum liquid is compared to water. It is expressed in degrees on the API scale. Higher API gravity values indicate lighter and less dense liquids, while lower values indicate heavier and denser liquids. The API gravity scale is inversely related to density, meaning higher API gravity corresponds to lower density.
Specific Gravity:
Definition: Specific gravity is a dimensionless ratio that compares the density of a substance to the density of a reference substance, usually water. It provides a measure of how much denser or lighter a substance is compared to water. For liquids, specific gravity is typically measured at a standard temperature. In the context of oil and gas, specific gravity is often used to characterize the density of hydrocarbons relative to water.
Acid Gas vs. Sour Gas:
Acid Gas:
Definition: Acid gas refers to a type of gas that contains acidic components, typically hydrogen sulfide (H2S) and/or carbon dioxide (CO2). These gases can be corrosive and pose safety and environmental hazards. The term is often used in the context of natural gas processing and refining industries, where the removal of acid gases is crucial to meet product specifications and safety standards.
Sour Gas:
Definition: Sour gas is natural gas or any other gas that contains a significant amount of hydrogen sulfide (H2S) and/or carbon dioxide (CO2). The term “sour” is used to indicate the presence of these acidic compounds. Sour gas requires special handling and processing to remove or reduce the acidic components, as they can be corrosive and pose health risks to both humans and the environment. Sour gas is contrasted with “sweet gas,” which is natural gas that contains minimal or no sulfur compounds.
Hazardous Area Classification vs. Non-Hazardous Area:
Hazardous Area Classification (HAC):
Definition: Hazardous Area Classification involves the systematic assessment and classification of areas where the presence of flammable or explosive atmospheres may occur. This classification helps in identifying the level of risk and selecting appropriate equipment and safety measures for the given area.
Non-Hazardous Area:
Definition: Non-Hazardous Area refers to locations where the atmosphere does not contain, or is not expected to contain, hazardous concentrations of flammable gases, vapors, combustible dusts, or other substances that could pose a risk of fire or explosion. In these areas, standard industrial equipment and safety measures are typically sufficient.
Flash Drum vs. Knockout Drum:
Flash Drum:
A flash drum is a vessel used in oil and gas processing to separate a mixture of liquid and vapor components by exploiting the differences in their volatility.
The process involves feeding a high-pressure liquid stream (often a mixture of hydrocarbons) into the flash drum, where it undergoes a sudden drop in pressure. This pressure reduction causes some of the liquid to vaporize or “flash” into vapor and separates from the remaining liquid.
The vapor phase, which is typically lighter hydrocarbons or gases, is then separated from the liquid phase. This separation is based on the principle that the more volatile components will vaporize at the given pressure drop.
Knockout Drum:
A knockout drum, also known as a liquid knockout drum or demister, is a vessel used to remove liquid droplets from a gas stream. It is often employed downstream of processes such as flash drums, compressors, or separators where gas and liquid may still be mixed.
The knockout drum uses gravity and sometimes additional devices such as demister pads or baffles to allow liquid droplets to settle and be separated from the gas stream. The separated liquid is typically collected at the bottom of the drum and drained off, while the gas is released from the top.
The purpose of a knockout drum is to protect downstream equipment, such as compressors or pipelines, from potential damage caused by liquid carryover.
Viscosity vs. Density:
Viscosity:
Definition: Viscosity is a measure of a fluid’s resistance to flow or deformation. It describes how easily a fluid can be stirred or moved, and it is often referred to as the thickness or stickiness of a fluid.
Symbol: The unit of viscosity is typically measured in pascal-seconds (Pa·s) or poise (P).
Density:
Definition: Density is a measure of mass per unit volume of a substance. It quantifies how much mass is contained in a given volume of a substance. In the context of fluids, it indicates how much matter is packed into a specific space.
Symbol: The unit of density is typically measured in kilograms per cubic meter (kg/m³) or grams per cubic centimeter (g/cm³).
Heat Recovery Steam Generator (HRSG) vs. Boiler:
Heat Recovery Steam Generator (HRSG):
A Heat Recovery Steam Generator (HRSG) is a device that recovers heat from a hot gas stream generated during the combustion of a fuel or the utilization of industrial processes. The primary purpose of an HRSG is to capture the waste heat from these processes and use it to generate steam, which can then be used for various applications, such as power generation or industrial heating. HRSGs are commonly used in combined cycle power plants, where they recover heat from the exhaust gases of a gas turbine to produce additional steam for a steam turbine, thereby improving overall efficiency.
Boiler:
A boiler is a closed vessel or arrangement of vessels and tubes used to convert water into steam by heating it through the combustion of a fuel or the application of heat from an external source. The steam produced by a boiler can be used for various purposes, including power generation, heating, and industrial processes. Boilers come in different types and designs, such as fire-tube boilers and water-tube boilers, and they play a crucial role in a wide range of industries where steam is an essential component of the production process or for space heating.
Fluid Flow vs. Mass Flow:
Fluid Flow:
Fluid flow refers to the movement of a substance (fluid) from one point to another within a system. This substance can be a liquid or a gas. Fluid flow is characterized by the dynamic behavior of the fluid, and it can be influenced by factors such as pressure, velocity, viscosity, and the geometry of the system through which the fluid is flowing.
Mass Flow:
Mass flow is a measure of the mass of a substance that moves through a system per unit of time. It is expressed in terms of mass per unit time, typically in kilograms per second (kg/s) or other mass units per unit time. Mass flow takes into account the density of the substance and the velocity of its flow. In the context of fluid flow, mass flow is often considered to be a more fundamental parameter than volumetric flow, as it directly accounts for the amount of material being transported.
Molten Salt vs. Molten Metal:
Molten Salt:
Definition: Molten salt refers to a state in which a salt compound, typically ionic in nature, has been heated to the point where it transforms from a solid crystalline structure to a liquid form. In this state, the ions within the salt are free to move, giving the substance a fluid-like consistency.
Molten Metal:
Definition: Molten metal refers to the liquid state of a metallic substance when it is heated to a temperature above its melting point. In this state, metallic bonds are weakened, allowing the metal atoms to move more freely. This liquid metal can be poured or shaped into different forms before solidifying again upon cooling.
Cryogenic vs. Ambient Temperature:
Cryogenic Temperature:
Definition: Cryogenic temperature is extremely low temperature, typically below -150 degrees Celsius (-238 degrees Fahrenheit) or even lower. Common gases like nitrogen and oxygen become liquids at cryogenic temperatures. Cryogenic conditions are often used in various scientific, industrial, and medical applications, such as in the storage and transportation of liquefied gases.
Ambient Temperature:
Definition: Ambient temperature is the temperature of the surrounding environment or the immediate surroundings. It is the temperature that is naturally present in a particular space. For most day-to-day purposes, ambient temperature refers to the normal or room temperature conditions that people typically experience in their daily lives. It is usually within the range of 20 to 25 degrees Celsius (68 to 77 degrees Fahrenheit).
Safety Integrity Level (SIL) vs. Risk Assessment:
Safety Integrity Level (SIL):
Definition: Safety Integrity Level (SIL) is a measure of the effectiveness of a safety system in achieving functional safety. It is a quantitative measure of the reliability of a system to perform a specific safety function under prescribed conditions. SIL is used to assess and mitigate risks associated with potential hazards in industrial processes. The higher the SIL, the lower the probability of a system failure leading to a hazardous event.
Risk Assessment:
Definition: Risk assessment is a systematic process of identifying, evaluating, and prioritizing potential hazards or risks within a system, process, or activity. It involves the analysis of the likelihood and consequences of various hazardous events. The goal of risk assessment is to provide a basis for making informed decisions about risk mitigation and management strategies. This process typically includes the identification of hazards, determination of the likelihood and severity of their consequences, and the evaluation of existing control measures.
Distillation Column vs. Absorption Column:
Distillation Column:
A distillation column is a vertical industrial tower used to separate components in a liquid mixture based on their different boiling points. It operates on the principle of fractional distillation, where the liquid mixture is heated to create vapor, and then the vapor is condensed back into liquid form. The components with higher boiling points condense at lower sections of the column, while those with lower boiling points rise to higher sections, resulting in the separation of the components.
Absorption Column:
An absorption column is a vertical vessel designed to absorb a gas component into a liquid phase. In this process, a gas stream is brought into contact with a liquid absorbent, allowing the transfer of one or more components from the gas phase to the liquid phase. The absorption column is commonly employed in various industrial applications, such as gas purification and removal of specific components from gas streams. The liquid absorbent selectively captures certain gases, leading to separation and purification.
Catalytic Cracking vs. Hydrocracking:
Catalytic Cracking Definition:
Catalytic cracking is a refining process in which large hydrocarbon molecules, typically found in heavy crude oil fractions or residues, are broken down into smaller, more valuable products. This is achieved by using a catalyst, usually solid particles like zeolites, to promote the cracking reactions. The process enhances the production of gasoline and other high-demand products.
Hydrocracking Definition:
Hydrocracking is a refining process that involves the use of hydrogen and a catalyst to break down complex hydrocarbons into lighter and more valuable products. Unlike catalytic cracking, hydrocracking is conducted in the presence of hydrogen gas, which helps to saturate the unsaturated hydrocarbons and reduce the formation of coke. This process is particularly effective in converting heavy feedstocks into high-quality products such as diesel, jet fuel, and lubricating oils.
Turbulent Flow vs. Laminar Flow:
Turbulent Flow: Chaotic fluid motion characterized by irregular fluctuations in velocity and pressure.
Laminar Flow Definition:
Laminar flow is a smooth, orderly flow of fluid in which the layers of the fluid move in parallel, without significant mixing between adjacent layers. The flow is characterized by streamlined, well-defined paths, and the fluid particles move in a regular manner.
Turbulent Flow Definition:
Turbulent flow is a chaotic and irregular flow pattern in which the fluid undergoes rapid changes in velocity and pressure. Unlike laminar flow, turbulent flow is characterized by eddies, swirls, and fluctuations in velocity. The fluid particles mix vigorously, resulting in a less predictable and more disorderly flow.
Reboiler vs. Condenser:
Reboiler Definition
A reboiler is a heat exchanger used in a distillation column to provide the necessary heat to vaporize the liquid entering the bottom of the column. It functions by taking a portion of the liquid from the bottom of the column (usually the product stream) and heating it, causing it to vaporize. The resulting vapor is then returned to the bottom of the column to enhance the separation of components. Reboilers play a crucial role in maintaining the distillation process by ensuring that the liquid entering the column is sufficiently vaporized for effective separation.
Condenser Definition
A condenser is another heat exchanger in a distillation column, but its purpose is to cool and condense the vapor leaving the top of the column into a liquid phase. As the vapor rises in the column and becomes enriched with the desired components, it reaches the condenser, where it is cooled by either air or water. This cooling causes the vapor to condense into a liquid. The condensed liquid is then collected as a distillate product, and any remaining vapor is sent back into the column for further separation.
Stainless Steel vs. Carbon Steel:
Stainless Steel Definition:
Stainless steel is a type of steel alloy containing at least 10.5% chromium. The presence of chromium in the alloy creates a protective layer of chromium oxide on the surface, which makes it highly resistant to corrosion, staining, and rusting. Stainless steel is known for its durability, strength, and aesthetic appeal. It is widely used in various applications, including kitchen appliances, cutlery, construction, and medical instruments.
Carbon Steel Definition:
Carbon steel is a steel alloy that primarily consists of iron and carbon, with the carbon content typically ranging from 0.05% to 2.0%. Carbon steel may also contain other elements, such as manganese, silicon, and copper, in varying amounts. The presence of carbon provides hardness and strength to the steel. Carbon steel is versatile and commonly used in a wide range of applications, including construction, manufacturing, and automotive industries. However, it is more susceptible to corrosion compared to stainless steel.
Batch Distillation vs. Continuous Distillation:
Batch Distillation Definition:
Batch distillation is a process in which a finite amount of a liquid mixture is heated and distilled in a single batch or lot. The mixture is introduced into a distillation apparatus, heated to vaporize its components, and then the vapor is condensed and collected. This method is suitable for smaller-scale production and is characterized by discontinuous operation.
Continuous Distillation Definition:
Continuous distillation is a process in which a liquid mixture is continuously fed into a distillation column or system, and the separation of components occurs continuously over time. It involves a steady input of the feedstock and the continuous removal of the products and by-products. Continuous distillation is often employed in large-scale industrial processes for its efficiency and the ability to handle large quantities of materials.
Occupational Exposure Limit (OEL) vs. Permissible Exposure Limit (PEL):
Occupational Exposure Limit (OEL): Definition:
Occupational Exposure Limits are concentrations of airborne contaminants in the workplace that are believed to be safe for nearly all workers over a working lifetime. These limits are established to protect workers from adverse health effects associated with exposure to various substances such as chemicals, dust, or biological agents.
Permissible Exposure Limit (PEL) Definition:
Permissible Exposure Limits are specific occupational exposure limits set by regulatory authorities, such as the Occupational Safety and Health Administration (OSHA) in the United States. PELs represent the maximum allowable concentrations of hazardous substances in the air, averaged over a specified time period, to which workers may be exposed without experiencing adverse health effects.
Alkylation vs. Dealkylation:
Alkylation Definition:
Alkylation is a chemical reaction in which an alkyl group is added to a molecule, typically replacing a hydrogen atom. This process often involves the combination of an alkylating agent (a compound that provides the alkyl group) and a substrate molecule.
Dealkylation Definition:
Dealkylation is a chemical reaction in which an alkyl group is removed from a molecule, resulting in the formation of a new compound and the release of an alkyl fragment. This process often involves the cleavage of a carbon-alkyl bond within a molecule.