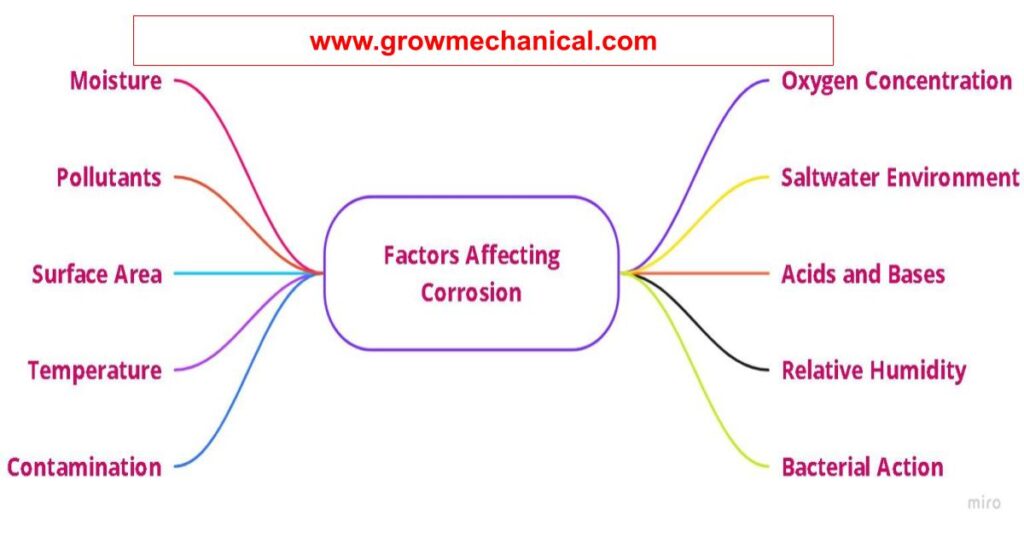
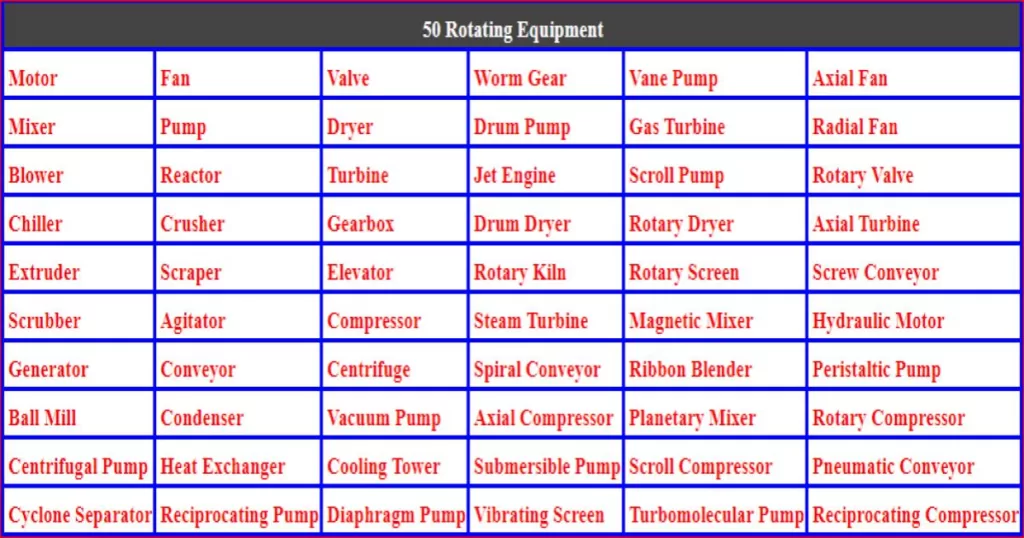
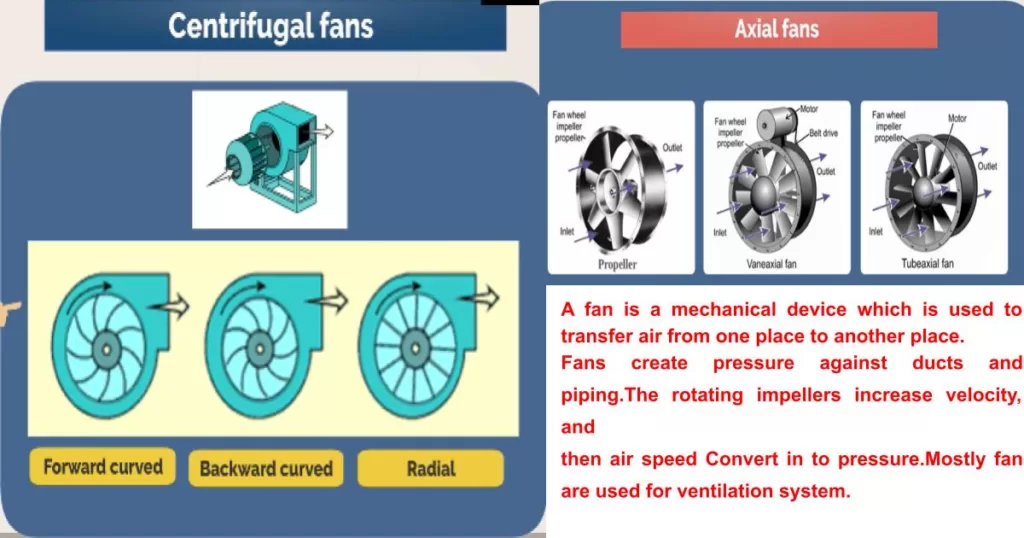
Factors Affecting Corrosion
Corrosion is a process of deterioration in metals caused by chemical, electrochemical, and biochemical reactions. It can significantly reduce the life of a metal structure or component. Different environmental factors, such as oxygen concentration, temperature, and surface area, all contribute to corrosion. This article provides an overview of the top 8 factors that can affect corrosion.
Oxygen Concentration
Oxygen concentration is one of the most important factors affecting corrosion. Oxygen molecules are attracted to metal surfaces and provide the energy required for oxidation reactions. The higher the oxygen concentration in a given environment, the greater its corrosive tendency. As more oxygen molecules react with the metal surface, they create a corrosion layer that can lead to metal degradation and component failure over time.
Oxygen can cause potential damage to metals since it can create an electric circuit on a metallic surface that accelerates corrosive reactions with other materials like iron oxide, carbon steel, aluminum, or magnesium alloys. In general oxygen needs a high humid rich environment for successful corrosion reaction.
Temperature
Temperature also plays an important role in corrosion. High temperatures can accelerate the rate of corrosion and cause materials to become more brittle. Low temperatures can slow the corrosive reaction down, but they are not entirely protective. Ice is still a long-term corrosive element in many environments and cold weather conditions can have similar effects as extreme heat. The temperature should be monitored closely to ensure optimal environment factors for a particular metal or structure.
Corrosion depends on a few different temperatures primarily being ambient temperature as well as temperatures in regards to exposure time intervals and exposure conditions (fumes or other chemical agents). In general, corrosion is accelerated at higher temperatures due to faster chemical reactions taking place between an electrolyte (salt solution) and the metal. This is why hot water tanks are made with special metals that are designed to resist corrosion.
Surface Area
The rate of corrosion is also governed by the surface area of a metal or structure. A larger surface area exposed to the corrosive elements will cause more corrosion than a smaller one. This means that objects with rough surfaces corrode faster than smooth ones, as there is more surface area for the element to react with. For this reason, protective barriers are often used to limit the amount of exposed material, such as paint or an outer layer of metal plating.
Contamination from Other Elements
Corrosion can also be caused by contamination from other elements, such as sulfur and ammonia. When these elements are present in the environment, they can react with metal surfaces to form a corrosive film. This can lead to the metal corroding and weakening over time. It is important to ensure that proper protective measures are taken to prevent contamination from occurring in the first place.
Relative Humidity
Relative humidity plays a big role in the corrosion process, particularly when it comes to the amount of water that is present in the air. High levels of moisture can increase the rate of corrosion, since water has a greater ability to penetrate metals and react with them than dry air does. The formation of rust on metal surfaces is also accelerated significantly when relative humidity is above 55%.
Moisture
One of the most significant and common environmental factors that causes corrosion is moisture. Any type of aqueous environment, such as water or humid air, increases the rate of corrosion when it comes into contact with metal surfaces. The moisture will cause oxidation, which further weakens the metal surface and leads to corroding.
Acids and Bases
Acids and bases affect different types of metals differently when they come into contact with them causing some metals to corrode, while others may remain unaffected by their presence in certain cases depending upon the amount used and various other environmental factors like humidity levels etcetera
Pollutants
Air pollution has been found to contribute significantly to the corrosion process by providing an additional source of sulfur dioxide (SO2) which then combines with atmospheric water creating sulfuric acid mist which affects any kind of unprotected metallic surface leading onto immediate corrosion effects over time if not addressed immediately by proper mitigation methods.
Bacterial Action
Bacteria can play a major role in contributing towards faster rates of corrosion since its metabolic activities produce compounds that are acidic which then accelerates the process leading to higher chances for perforation or breakage within any structural system using potentially weakened materials from microbial degradation.
Saltwater Environment
Saltwater environments present one of the most vulnerable conditions for any type of exposed metallic surface since salinity needs very little extra added components for serious corrosive impacts over time. This usually directly affects coastal area infrastructures exposing them direct to heavy actions. Forms incorporating naturally formed salts find its way toward magnifying corrosive agents
Corrosion prevention
Cathodic Protection
Cathodic protection (CP) is a method of corrosion prevention that uses electrical current to reduce the potential between an anode and metal surface, which can in turn prevent corrosion. Unlike protective coatings, CP systems prevent metal erosion by preventing the oxidation reaction from happening. This type of protection works best on metal structures located in or near air, water, and soil contact.
Protective Coatings
Protective coatings are special materials that are applied to metal surfaces to protect them from chemicals, heat, sunlight, weathering, and more. They act as a barrier between the metal surface and the corrosive elements present in its environment and provide added protection against damage caused by oxidation. Additionally, they often come with additional benefits like improving appearance or reducing maintenance requirements.
Environmental Factors
Corrosion of metals can also be prevented by controlling factors such as humidity levels, temperature ranges, exposure to saltwater spray or condensation, pollutants in outdoor air sources like smog and acid rain etc. Outdoors above-ground storage tanks are some examples where environmental conditions can have a big negative impact on metals’ durability over time if not properly maintained.
Preventative Maintenance
Regular inspections for signs of corrosion can help identify problem areas before extensive damage takes place so that correct measures can be taken to remedy them accordingly – this is known as preventative maintenance or PM for short; it is one of several proactive strategies used to avoid breakdowns before they occur and minimize downtime due to unexpected problems resulting from wear-and-tear of industrial components within large manufacturing operations like automotive factories etc.
Alloys Resistance
The addition of various alloying elements like chromium, nickel or molybdenum can greatly improve the resistance of certain metals against corrosion process due to formation of special oxide layers that form over their surfaces when exposed to moist environments – these alloys are primarily used in resistant applications including pipe systems found deep undergrounds as well as ocean vessels, which must endure intense underwater corrosive conditions as part of their daily operations respectively!
Reduction Of Chlorides And Oxygen
Another way to reduce corrosion is to reduce chloride content in the environment, because chloride ions act as catalysts during the corrosion process. Also, we might decrease oxygen levels in the environments because oxygen attacks specific metals when combined with which other elements inside the environment, for example aluminum, corrode faster when exposed to atomic hydrogen.
Regular Cleaning:
Regular cleaning means removal of dirt. The dirt build up will increase the pace of the corrosion process, so it is important to take off those buildups by regular cleaning sessions.
- TYPES OF PIPE FLANGE
- SCREWED FLANGE
- SLIP ON FLANGE
- SOCKET WELD
- SORF FLANGE
- NIPO FLANGE
- LAP JOINT